QPCTL
A Small Molecule Inhibitor of QPCTL as a Potential Treatment of Tumors with High Engagement
of CD47-Signal Regulatory Protein α ("SIRPα") Axis
(Phase I)
of CD47-Signal Regulatory Protein α ("SIRPα") Axis
(Phase I)
Assays Completed
Co-development with Fosun Pharma
- In vivo anti-tumor
efficacy studiesCombo study with Rituximab, anti-PD-1, or paclitaxel, Azacytidine -
 In vivo efficacy studies
In vivo efficacy studies
with single agent -
 In vivo PK-PD
In vivo PK-PD -
 In vivo PK studies
In vivo PK studies - In vivo acute efficacy studies
-
 In vitro ADMET studies
In vitro ADMET studies -
 Developability/CMC
Developability/CMC -
 In vitro cell-based
In vitro cell-based -
 Toxicology studies
Toxicology studies - Enzymatic
Target Rationale
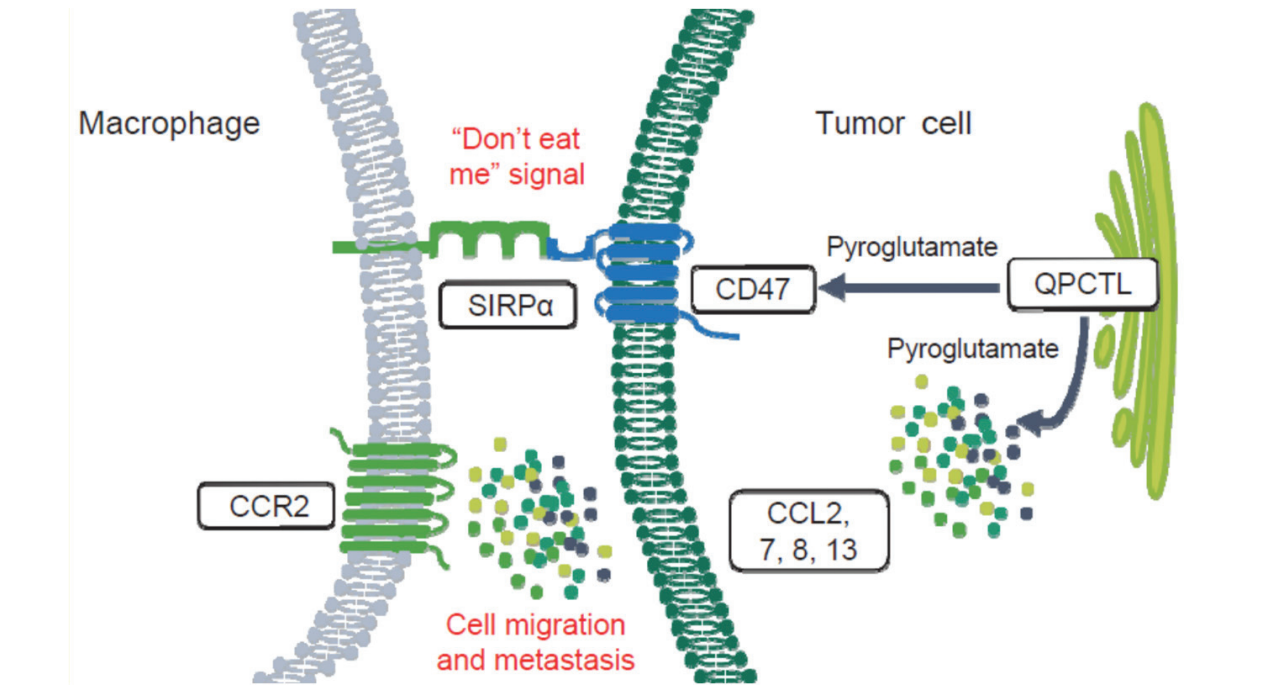
QPCTL, which is located on the cell organelle called the Golgi apparatus, has been identified as a crucial regulator of the CD47-SIRPα axis, commonly referred to as the "don't eat me" axis.
Inhibition of QPCTL with pharmacological tools or bioengineered knockout methods has been shown to cause a reduction or loss of the binding between CD47 and SIRPα, as well as increased antibody-dependent cellular phagocytosis and neutrophils-induced cytotoxicity. Given the lack of Golgi apparatus where QPCTL is localized in mature erythrocytes, QPCTL inhibitors may induce lower hematological toxicity compared to most of CD47-blocking agents currently under clinical development.
In addition, downregulation of CCL2/CCR2 signaling transduction by inhibiting QPCTL could be used to reprogram the tumor immune microenvironment through modulating suppressive myeloid cells toward phagocytic macrophages-enriched profile, subsequently turn less T cell-inflamed tumors into highly T cell-infiltrating tumor, and further favor anti-tumor immunity led by T cell engagers like anti-PD-1/L1 antibodies.
Inhibition of QPCTL with pharmacological tools or bioengineered knockout methods has been shown to cause a reduction or loss of the binding between CD47 and SIRPα, as well as increased antibody-dependent cellular phagocytosis and neutrophils-induced cytotoxicity. Given the lack of Golgi apparatus where QPCTL is localized in mature erythrocytes, QPCTL inhibitors may induce lower hematological toxicity compared to most of CD47-blocking agents currently under clinical development.
In addition, downregulation of CCL2/CCR2 signaling transduction by inhibiting QPCTL could be used to reprogram the tumor immune microenvironment through modulating suppressive myeloid cells toward phagocytic macrophages-enriched profile, subsequently turn less T cell-inflamed tumors into highly T cell-infiltrating tumor, and further favor anti-tumor immunity led by T cell engagers like anti-PD-1/L1 antibodies.
Insilico Medicine QPCTL Inhibitor Summary –Phase I
Novel structure generated by AI
- Distinctly different structure generated by Insilico Medicine's AI small molecule generation platform Chemistry42
Effective Cancer Immuno-therapy
- Potent inhibitory activity of SIRP-α binding to CD47-expressing cancer cells
- Novel I/O class utilizing innate immunity and further boosting adaptive immunity through reprogramming tumor myeloid landscape toward anti-tumorigenic profile
- Potential broad indications including both NHL/AML and solid tumor
Promising drug-ability as an oral agent
- Good in vitro ADME profiles
- Promising PK profiles across different preclinical animal species
Favorable safety margin
- Promising MOS in rat and dog based on GLP studies without obvious off-target toxicity
- Clean on Safety Pharmacology
Indication
The detection and clearance of cancer cells via phagocytosis induced by innate immune checkpoints plays a significant role in tumor-mediated immune escape. The CD47-SIRPα axis, whose signaling is sustained by QPCTL, is the most well-described innate immune checkpoint which allows the CD47-expressing cancer cells to evade innate immune cell-mediated phagocytosis. The agents that block the CD47–SIRPα interaction are currently being evaluated in multiple ongoing trials, most in combination with pro-phagocytosis agents, e.g. therapeutic antibodies, chemotherapy in patients with leukemia, lymphoma and advanced solid tumors (head and neck squamous cell carcinoma, gastric cancer, breast cancer, colorectal cancer, etc.). The QPCTL inhibitor could be developed for the patients with these indications, especially when they are resistant to the standard-of-care therapies, e.g. azacytidine, rituximab, paclitaxel, trastuzumab, cetuximab, etc.
Project Status – Phase I
Currently co-developed in partnership with Fosun, a first-in-class orally available small molecule inhibitor of QPCTL, a regulator of the CD47-SIRPα axis, as cancer immunotherapy. The preliminary results demonstrated potent enzymatic inhibitory activity, strong efficacy and synergistic effects with other therapies. We filed an IND application with the NMPA in China in April 2023 and expect to initiate Phase I clinical trial in the second half of 2023.
Discovered by leveraging our Pharma.AI platform. We utilized PandaOmics' target identification and business intelligence capabilities to identify novel oncology targets, and QPCTL was listed among the promising novel druggable targets with implications in the CD47-SIRPα axis. We then used Generative Chemistry to generate the candidate against QPCTL for treatment of tumors with high engagement of CD47-SIRPα axis.
Discovered by leveraging our Pharma.AI platform. We utilized PandaOmics' target identification and business intelligence capabilities to identify novel oncology targets, and QPCTL was listed among the promising novel druggable targets with implications in the CD47-SIRPα axis. We then used Generative Chemistry to generate the candidate against QPCTL for treatment of tumors with high engagement of CD47-SIRPα axis.

Synergistic inhibitory effect on tumor growth when combined with tumor-opsonizing antibodies
