PHD1/2 — IBD
A Small Molecule Inhibitor of PHD1/2 for Treatment of Inflammatory Bowel Disease (Phase IIa Ongoing)
Assays Completed
Wholly-owned and Available for Licensing
- In vivo efficacy studiesCombo study with Mesalamine,
Anti-TNFα antibody and CsA - In vivo efficacy studies
with single agent -
 In vivo PK studies
In vivo PK studies -
 In vitro ADME studies
In vitro ADME studies -
 Developability/CMC
Developability/CMC -
 In vitro cell-based
In vitro cell-based -
 Toxicology studies
Toxicology studies - Enzymatic
Target Rationale
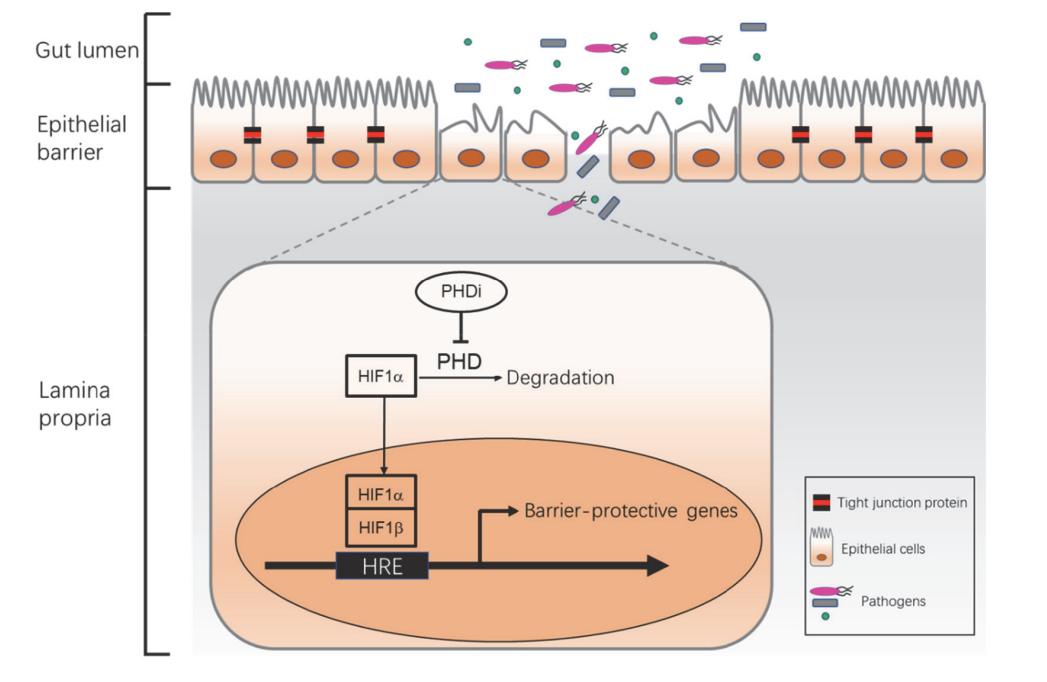
HIFs have more recently been recognized as a protective regulator of IBD by binding HRE and driving the expression of barrier protective genes. PHDs are responsible for hydroxylation of HIFα, which initiates the pathway that eventually results in the degradation of HIFα by the proteasome.
Inhibition of PHDs, especially PHD1 and PHD2, reduces the degradation of HIFα and higher level of HIFs leads to barrier-protective gene expression for epithelial barrier healing and decreased proinflammatory cytokine expression. However, because HIF transcription factors are involved in the regulation of broad biological processes, systematic exposure to PHD inhibitors may increase the risk of malignancy, retinopathy, thrombosis, hyperkalemia, and elevated blood pressure. Therefore, oral delivery of gut-restricted PHD1 and PHD2 dual inhibitors may be an innovative clinical strategy for IBD patients.
Inhibition of PHDs, especially PHD1 and PHD2, reduces the degradation of HIFα and higher level of HIFs leads to barrier-protective gene expression for epithelial barrier healing and decreased proinflammatory cytokine expression. However, because HIF transcription factors are involved in the regulation of broad biological processes, systematic exposure to PHD inhibitors may increase the risk of malignancy, retinopathy, thrombosis, hyperkalemia, and elevated blood pressure. Therefore, oral delivery of gut-restricted PHD1 and PHD2 dual inhibitors may be an innovative clinical strategy for IBD patients.
Insilico Medicine PHD1/2 — IBD Inhibitor Summary –
Phase I Completed
Phase I Completed
Novel structure generated by AI
- Distinctly different structure generated by Insilico Medicine's AI small molecule generation platform Chemistry42
Effective in both mono and combo therapies
- Potent PHD1/2 enzymatic inhibition
- Potent HIF-1α induction
- Efficacious monotherapy in both TNBS and DSS colitis Model
- Efficacious combo-therapy with anti-inflammatory drugs in both TNBS and DSS colitis model
Promising drug-ability as an oral agent
- Good in vitro ADME profiles
- Promising gut-restricted PK profiles across different preclinical animal species
Favorable safety margin
- Promising MOS in rat and dog studies
- Favorable in vitro safety profiles
Indication
Inflammatory bowel disease (IBD) is characterized by the inflammation of the colon and small intestine, leading to ulcerative colitis, and Crohn's disease. Ulcerative colitis primarily affects the colon and the rectum, whereas Crohn's disease affects the whole gastrointestinal tract. These diseases are characterized by chronic inflammation and wounding of the mucosa and loss of the intestinal epithelial barrier function.
Project Status – Phase I Completed
An oral, gut-restricted small molecule inhibitor of PHD1/2 for the treatment of IBD, which is an umbrella term for disorders that involve chronic inflammation of the digestive tract, such as ulcerative colitis and Crohn's disease. The preclinical studies show that the candidate is well tolerated and demonstrates efficacy in several murine colitis models induced by different chemicals. We have initiated IND-enabling studies and expect to file our IND applications in China, the U.S. in the second half of 2023.
Discovered by leveraging our PHARMA.AI platform.
Discovered by leveraging our PHARMA.AI platform.

ISM compound demonstrated significant therapeutic efficacy in both TNBS and DSS induced IBD mouse models
PHD1/2 — CKD
A Small Molecule Inhibitor of PHD1/2 as a Potential Treatment of Anemia of CKD (IND Clearance)
Assays Completed
Great China Rights
Out-licensed to TaiGen
-
 In vivo efficacy studies with single agent
In vivo efficacy studies with single agent -
 In vivo PK-PD
In vivo PK-PD -
 In vivo PK studies
In vivo PK studies -
 In vitro ADME studies
In vitro ADME studies -
 Developability/CMC
Developability/CMC -
 In vitro cell-based
In vitro cell-based -
 Toxicology studies
Toxicology studies - Enzymatic
Target Rationale
Anemia of CKD is a form of normocytic normochromic, hypoproliferative anemia. The final molecular step that controls HIFs stabilization converges on prolyl hydroxylases ("PHDs"). PHDs hydroxylates HIFα, a subunit of HIF, leading to degradation of HIFα by the proteasome. Among three subtypes of PHDs, namely PHD1, PHD2 and PHD3, PHD2 demonstrates more abundant expression across tissue types compared to the other subtypes and inhibition of PHD2 has been indicated as a promising therapy for HIFα-related diseases.
Inhibition of PHD1/2 might upregulate the expression of several iron metabolism genes, such as divalent metal transporter 1 ("DMT1") and duodenal cytochrome b, which have been demonstrated in preclinical studies of PHD1/2 inhibitors conducted by others. Thus, inhibition of PHD1/2 addresses both impaired EPO production and functional iron deficiency.
Inhibition of PHD1/2 might upregulate the expression of several iron metabolism genes, such as divalent metal transporter 1 ("DMT1") and duodenal cytochrome b, which have been demonstrated in preclinical studies of PHD1/2 inhibitors conducted by others. Thus, inhibition of PHD1/2 addresses both impaired EPO production and functional iron deficiency.

Insilico Medicine PHD1/2 — CKD Inhibitor Summary –
IND Clearance
IND Clearance
Novel structure generated by AI
- Distinctly different structure generated by Insilico Medicine's AI small molecule generation platform Chemistry42
Effective in both mono and combo therapies
- Potent PHD1/2 enzymatic inhibition
- Potent HIF-1α induction
- Potent EPO induction activity
- Lower efficacious dose in rat CKD model
- Achieve target HGB level via lower EPO induction in rat CKD model
Promising drug-ability as an oral agent
- Good in vitro ADME profiles
- Promising PK profiles across different preclinical animal species
- No hits on KinomeScan and Cerep panels
Favorable
safety margin
safety margin
- Favorable in vitro safety profiles
Indication
Anemia of CKD is a form of normocytic normochromic, hyperproliferative anemia. The pathogenesis of anemia is impaired EPO production and functional iron deficiency. EPO is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia, in order to stimulate red blood cell production (erythropoiesis) in the bone marrow.
Widespread use of erythropoietin stimulating agents (ESA) improved overall quality of life and reduced the need for blood transfusions. Liberal administration of ESA was associated with increased risk of cardiovascular events, CKD progression, vascular access thrombosis, and overall mortality. Additional issues include, high cost of EPO analogs and associated resistance as well as side effects.
Alternatively, PHD inhibitors can be used to treat anemia by enhancing EPO secretion via upregulation of HIF-1α.
Widespread use of erythropoietin stimulating agents (ESA) improved overall quality of life and reduced the need for blood transfusions. Liberal administration of ESA was associated with increased risk of cardiovascular events, CKD progression, vascular access thrombosis, and overall mortality. Additional issues include, high cost of EPO analogs and associated resistance as well as side effects.
Alternatively, PHD inhibitors can be used to treat anemia by enhancing EPO secretion via upregulation of HIF-1α.
Project Status – IND Clearance
An oral small molecule inhibitor of PHD1/2 for the potential treatment of anemia of CKD. The preclinical studies show that the cadidate is well tolerated and demonstrates high potency to rescue anemia in CKD rats. We filed our IND application in China in June 2023 and expect to initiate Phase I clinical trial in late 2023.
Discovered by leveraging our Pharma.AI platform. Engaging the Generative Chemistry application through structure-based drug design, small molecules were generated and prioritized targeting PHD1/2. Discovered as a preclinical candidate for the potential treatment of anemia of CKD after several rounds of optimization on physicochemical properties as well as in vitro and in vivo absorption, distribution, metabolism, and excretion ("ADME") profiles.
Discovered by leveraging our Pharma.AI platform. Engaging the Generative Chemistry application through structure-based drug design, small molecules were generated and prioritized targeting PHD1/2. Discovered as a preclinical candidate for the potential treatment of anemia of CKD after several rounds of optimization on physicochemical properties as well as in vitro and in vivo absorption, distribution, metabolism, and excretion ("ADME") profiles.

ISM compound demonstrated excellent efficacy in nephrectomy (NX) disease models
